Join Our Wellness Journey
Subscribe for exclusive tips and insights on enhancing your health.


SLU-PP-332 (250mcg x 60 Capsules = 15,000mcg)
SLU-PP-332 (1000mcg x 30 Capsules = 30,000mcg)
SLU-PP-332 is an experimental compound designed to mimic the metabolic effects of exercise by activating estrogen-related receptors (ERRs), particularly ERRα, ERRβ, and ERRγ. This activation leads to increased energy expenditure, enhanced fatty acid oxidation, and improved mitochondrial function.
Everyone knows that repetitive physical exercise leads to improved health and wellness. Research has repeatedly revealed that exercise can thwart heart disease, fight obesity, improve mood, boost cognitive function, and even help to prevent/treat a number of diseases. For the most part, attempts to replicate the benefits of exercise using pharmaceuticals has fallen short. Even weight loss drugs were a bust until the recent advent of peptides like semaglutide and liraglutide. A new breakthrough is underway, however, and researchers have developed a compound that can replicate some of the benefits of exercise. SLU-PP-332 is an estrogen-related receptor (ERR) agonist that binds primarily to ERR subclasses alpha and gamma. It has been shown to improve skeletal muscle endurance, boost weight loss, improve cardiovascular health, and protect the central nervous system against the ravages of aging and disease. SLU-PP-332 is the closest science has yet come to replicating the effects of exercise and has, predictably, generated a great deal of interest in research circles.
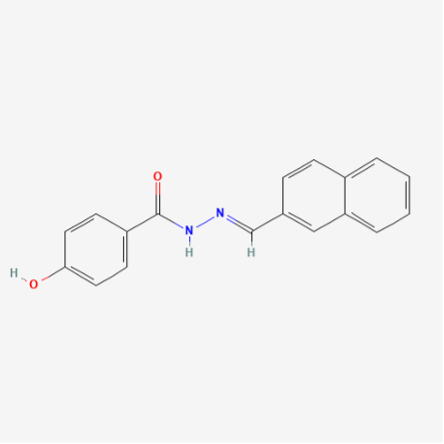
Chemical Formula: C18H14N2O2
Molecular Weight: 290.3 g/mol
PubChem CID: 5338394
CAS No.: 303760-60-3
Synonyms: 4-Hydroxy-N'-(naphthalen-2-ylmethylene) benzohydrazide
SLU-PP-332 is one of a family of compounds known as estrogen-related receptor agonists (ERRs). Research in animal models has revealed this compound to have a number of effects including:
Research indicates that SLU-PP-332 activates the estrogen receptor-related orphan receptors, which are called ERRs for short. ERRs are found with in nucleus of cells and their endogenous (natural) ligand has yet to be unambiguously identified, which is why they are referred to as “orphans.” These receptors come in three types as follows:
It is important to note that ERRs, despite their name, are not regulated by estrogen. The name arises from the fact that the gene for ERRα was first isolated due to homology to the gene for the estrogen receptor. That is where their similarities end, however, as evidence to date indicates that estrogen plays no role in the regulation of ERRs.
These receptors are known to regulate gene expression patterns with resulting impacts on energy homeostasis, oxidative metabolism, and mitochondrial biogenesis. Stimulation of these receptors can increase energy expenditure and fatty acid oxidation, leading to an increased rate fat loss. They also enhance mitochondrial function, particularly in heart and skeletal muscle cells to improve cardiovascular health as well as exercise tolerance [1].
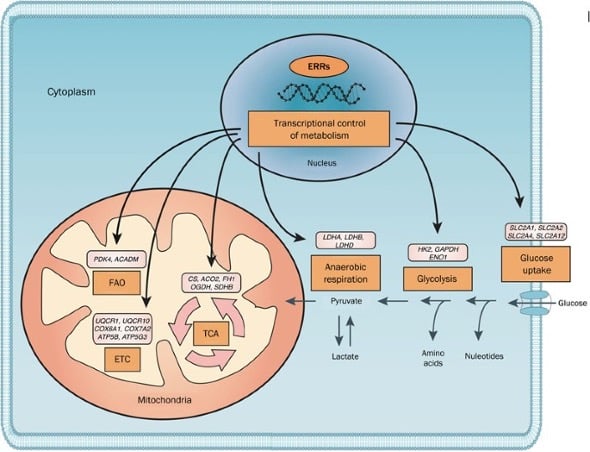
A look at the role ERRs play in the expression of genes that impact mitochondrial function
Source: Nature
One way in which scientists determined that ERRs are important to exercise tolerance is by creating mouse models, called knockouts, that lacked the ERR genes and thus ERRs in skeletal muscle. These mice showed profound intolerance to exercise. ERRα and ERRγ appear to be most important for exercise tolerance, with knockout mice for these two genes showing pale muscles under microscopic examination as well as severe exercise intolerance and decreased oxidative capacity. They also showed an inability to switch to lipid (fat) utilization which is critical for endurance exercise [2].
ERRα regulates genes involved in gluconeogenesis (the production of blood sugar from non-carbohydrate energy stores), fatty acid metabolism, and brown adipose tissue thermogenesis. It also regulates cholesterol, glucose, insulin, and triglyceride levels. It is a necessary receptor for responding to physiological and pathological stresses and has been shown to be a target of the statin class of drugs
ERRγ is much like ERRα. It is an important regulator of mitochondrial activity, plays a vital role in gene transcription, and is a major target of bisphenol A. Interestingly, the binding of bisphenol A (BPA) to the ERRγ receptor may be one reason that BPA has been linked to metabolic syndrome as well as cancer. BPA binding to ERRγ likely interferes with its ability to regulate mitochondrial activity leading directly to glucose dysregulation and, eventually, metabolic syndrome. BPA has long been known to be an endocrine disrupter, showing effects on bone strength and sexual development in mice [3]. Understanding the receptors to which BPA binds not only helps researchers to better counteract the effects of this well-known chemical, it provides deeper insight into mammalian development as well as the pathogenesis of certain disease conditions.
ERRγ is also being investigated for a potential role in the pathogenesis of Parkinson’s disease. It seems that Estrogen-related receptor gamma deficiency may hasten synuclein-mediated toxicity while overexpression can reduce inclusion body load and delay synuclein-mediated toxicity. Thus, ERRγ agonists, like SLU-PP-332 are under investigation as potential treatment in Parkison’s disease [4]. Mitochondrial dysfunction has long been thought to contribute to Parkinson’s disease and thus it makes sense that ERRγ has shown benefit in this condition.
ERRβ is a littler different from the other two ERRs, with its primary function seemingly being to regulate the transition of pluripotent stem cells from one state to another. Thus, ERRβ is likely important in tissue regeneration, growth, and development. It is the least well understood of the ERRs.
One of the things that makes SLU-PP-332 so important is its ability to reach ERRs throughout the body following injection. This is what allows SLU-PP-332 to be used in in vivo (inside living things) research. While ERR receptor agonists have been developed in the past, most of them were only useful for research in vitro (outside living things) because they were broken down or otherwise incapable of reaching their intended targets. SLU-PP-332 is one of the first compounds to have ERR-binding activity in actual living cells as well as an acceptable safety profile. Additionally, SLU-PP-332 is one of the first ERRα agonists developed. While ERRβ/γ agonists have been relatively easy to design, ERRα agonists have proven elusive. Until the development of SLU-PP-332, no ERRα agonist existed despite that fact that ERRα was the first ERR identified [5]. This compound has justifiably taken the exercise mimetic research field by storm.
The benefits of physical exercise are myriad. Research indicates that cardiovascular exercise, such as jogging, can enhance weight loss, boost muscle mass and function, increase bone strength, improve cardiovascular well-being, benefit the brain and central nervous system, and even slow down the process of aging [6]. The troubles with exercise, of course, are that not everyone finds it pleasant and making time for it can be difficult. For these, and other reasons, researchers have long searched for compounds that can simulate the benefits of exercise. Sometimes referred to as “exercise in a bottle,” the search for compounds that can mimic the beneficial effects of exercise has led to the discovery of the following compounds.
The peptides above represent just a sampling of the compounds that have been found to mimic some of the benefits of exercise without serious side effects. Prior to SLU-PP-332, very few compounds had been isolated that improved cellular respiration. Cellular respiration is the term used to describe the generation of energy by cells in the body. Most of this energy generation takes place with the mitochondria, which are contained within cells. Mitochondria respond very favorably to exercise, which not only increases the efficiency of these cellular power plants, but increases their numbers within the cell as well. Increases in mitochondria numbers and function are associated with increased basal metabolic rate, improved glucose tolerance, reduced insulin resistance, increased stamina, accelerated growth of small blood vessels, and emergent benefits like improved cardiovascular health.
SLU-PP-332 has been shown to directly impact the health of mitochondria [7], [8]. As a result of its impact on mitochondria, SLP-PP-332 has been shown to increase endurance and boost weight loss all without any changes in exercise habits for food intake. In fact, mice given SLU-PP-332 twice daily for a month lost a total of 12% of their body weight. Research in mice has even revealed that SLU-PP-332 can help to alleviate metabolic syndrome, which has heretofore proved very difficult to treat [5].
Research in mice shows that overexpression of ERRγ increases mitochondrial density. In other words, increased levels of ERRγ activity result in more mitochondria per cell. With more mitochondria, cells are able to utilize oxygen and nutrients faster and supply energy to the cell more efficiently. This means it takes longer for the cell to fatigue. In muscle, this means enhanced exercise capacity.
Interestingly, ERRγ also increases blood vessel density in skeletal muscle. This has two benefits. First, the increased blood vessel density means oxygen and nutrients can be more efficiently delivered to tissues and waste product can be more easily cleared. This, of course, boosts exercise tolerance. The second benefit is that increased blood vessel density is associated with increase insulin sensitivity and lower rates of diabetes [9].
So, exactly how effective is SLU-PP-332 in enhancing endurance? In normal-weight mouse models, SLU-PP-332 allowed the mice to run for 70% longer and 45% further than control mice [5]. This all results from an increase in the generation of energy at the cellular level, which allows muscles to remain in an aerobic state for longer periods of time. Of course, the muscles need to derive that energy from some stored source, which is why SLU-PP-332 can help to boost fat burning as well.
Research shows that ERR expression in skeletal muscle is induced in response to an exercise bout. Like many aspects of human physiology, ERR density responds to stress just as muscle mass responds to stress. Exercise creates an oxygen deficit in skeletal muscle cells, which leads those cells to produce more ERRs. This is the same as when lifting generates stress on skeletal muscle cells and leads those cells to generate more muscle fibers. SLU-PP-332 has the same effect on ERRs as exercise, leading to improved utilization of oxygen and nutrients by those muscles and thus improved muscle function [2].
SLU-PP-322 is sometimes referred to as a pan-ERR agonist because it has activity at all of the ERRs. In mouse models of cardiac failure (heart failure) SLU-PP-332 has been shown to improve ejection fraction, reduce rates of fibrosis, and increase survival in pressure overload-induced heart failure. The compound also helps to normalize fatty acid oxidation in the heart and improve energy homeostasis [10].
Fibrosis is extremely problematic in heart failure as it is a progressive problem that slowly replaces functional heart tissue with non-functional scar tissue. Fibrosis, which is another term for cardiac scarring, can lead to an inability of the heart to contract and expel blood properly and can even lead to cardiac conduction problems and arrhythmias. Thwarting fibrosis is one of the main objectives of care following the onset of heart failure. The ability to manage this particular complication of cardiac injury is critical to preserving heart function and, ultimately, to preventing cardiac death.
One of the ways in which SLU-PP-332 protects the heart against fibrosis is through regulation of autophagy. Autophagy is an essential recycling mechanism in many tissues that helps to remove diseased and damaged cells so that they can be replaced by healthy, functional cells. ERRs can induce autophagy through transcription factor EB (TFEB). By inducing TFEB activity, ERRs can increase autophagy and help to prevent the accumulation of damaged cells and scar tissue, allowing functional cells to thrive [11].
Parkinson’s disease is a neurodegenerative disorder characterized by the loss of dopaminergic neurons in a specific region of the brain called the substantia nigra pars compacta. Neurons in other areas of the brain can additionally show accumulation of a protein, alpha-synuclein, and the formation of Lewy bodies. Lewy bodies are associated with a specific type of dementia, referred to as Lewy-body dementia. It may or may not be accompanied by other Parkinson’s symptoms such as tremors and dyskinesia.
All neurons are exquisitely sensitive to oxidative stress, but research indicates that the neurons associated with Parkinson’s are especially vulnerable to oxidative stress and mitochondrial dysfunction. Even a momentary lapse in energy delivery to these neurons can have catastrophic consequences. Research in patients with Lewy-body disease shows a deficiency in expression for genes involved in mitochondrial respiration and function. This all suggests that while many different insults (e.g. stroke, chemical exposure, etc.) can lead to Parkinson’s, the common pathway is a lack of robust energy production by mitochondria. Thus, targeting mitochondrial function has long been a goal of Parkinson’s research.
As it turns out, ERRγ is required for maintaining mitochondrial content and the expression of genes related to autophagy, mitochondrial respiration and metabolism, synaptic vesicle cycling, transcriptional regulation, and vesicle-mediated transport. In other words, the very receptors that SLU-PP-332 targets play a critical role in regulating mitochondrial function in neurons as well as multiple other aspects of neuron function [4]. There is good reason to believe that ERR receptor agonists will provide hope for Parkinson’s sufferers in the future.
Declining renal function occurs with age, but severe loss of renal function is a consequence of disorders like hypertension and metabolic syndrome. It is thought that these conditions lead to inflammation in the kidney and eventual mitochondrial dysfunction, which is the ultimate cause of kidney disease. The kidneys are thus an excellent marker for overall aging and provide a good substrate organ for research into the effects of aging.
Research in human and mouse kidneys has shown that ERRs decrease with age except in individuals who were subjected to life-long calorie restriction. Calorie restriction is one of the few activities with proven anti-aging activity and is known to prolong both lifespan and healthspan. Mice subjected to calorie restriction show improved kidney function when compared to age-matched controls.
Based on this research, it is reasonable to conclude that ERR signaling is a critical factor in long-term well-being and, indeed, researchers have revealed that calorie restriction protects against age-related increases in albumin in the urine, a rise in inflammatory cytokines, and mitochondrial dysfunction. These same benefits are also seen in mice administered SLU-PP-332 that do not undergo calorie restriction [12]. In particular, activity at the ERRα has been shown to be most beneficial in mimicking the effects of calorie restriction and SLU-PP-332 is the only ERRα agonist available for in vivo testing.
It is important to note that mitochondrial dysfunction is one of the major hallmarks of aging and is closely interconnected with the process of cellular senescence. Decrease capacity of mitochondria to produce energy resulting in the production of oxygen free radicals which can be extremely damaging to cells. Free radicals lead to cellular damage and this, in turn leads to senescence or to cancer and other serious conditions when senescence is ineffective. The ability to protect mitochondrial function and thus reduce the generation of free radicals is a primary target of anti-aging researchers [13]. SLU-PP-332 is the first compound that has been shown to directly improve mitochondrial health.
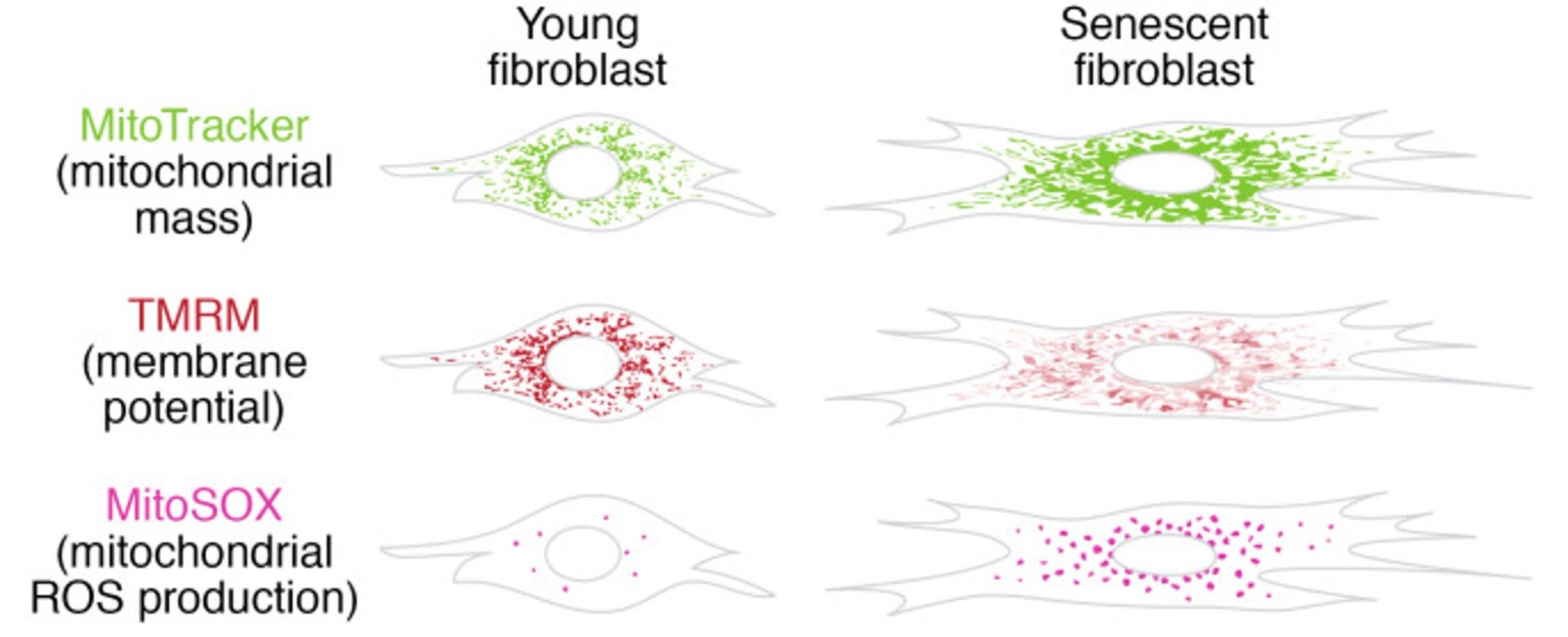
An illustration of mitochondrial reactive oxygen species (free radical) production in senescence.
Source: PubMed
SLU-PP-332 is one of three major ERR agonists currently under investigation. The other two are SLU-PP-1072 and SLU-PP-915. Of the two SLU-PP-1072 is the most similar to 332 in that it primarily interacts with the alpha and gamma ERR variants and has been shown to impact mitochondrial function in skeletal muscle. It has been investigated primarily for its ability to induce apoptosis in prostate cancer cells and is being pursued as a potential treatment for prostate cancer [14].
While similar to it companions, SLU-PP-915 has been primarily investigated for its benefits in heart failure. SLU-PP-915 is structurally distinct from 332, but both have been shown to improve cardiac ejection fraction, decrease fibrosis following cardiac injury, and increase survival rates in mouse models of heart failure. Both ERRα and ERRγ are important regulators of cardiac metabolism [15].
SLU-PP-332 is an estrogen-related receptor agonist with primary binding proclivities for ERRα and ERRγ. It has more limited binding to ERRβ. Early research has revealed that SLU-PP-332 acts primarily on mitochondria, boosting their capacity to generate energy and reducing oxidative stress as a result. This, in turn, results in increased exercise tolerance and endurance. Additional research has found that SLU-PP-332 can improve heart health, protect the kidneys from the effects of aging, and may help to thwart the pathogenesis of Parkinson’s disease. While research into SLU-PP-332 is just in its infancy, the compound has already shown some remarkable results and has opened up an entirely new area for exploration. SLU-PP-332 is helping scientists to better understand human physiology and will likely lead to the development of a number of therapeutic compounds in the future.